A thermometer is an instrument used to measure temperature. The commonly used thermometers include: mercury thermometers and alcohol thermometers.
Mercury and alcohol are the main components used to create thermometers, referred to as thermometric fluids. These fluids can measure temperature because they have the characteristic of expanding when heated and contracting when cooled. As the temperature increases, the volume of mercury and alcohol expands significantly. This can be observed in the thermometer as the height of the mercury column or the alcohol column rises. Therefore, by marking appropriate graduations, one can read the corresponding temperature.
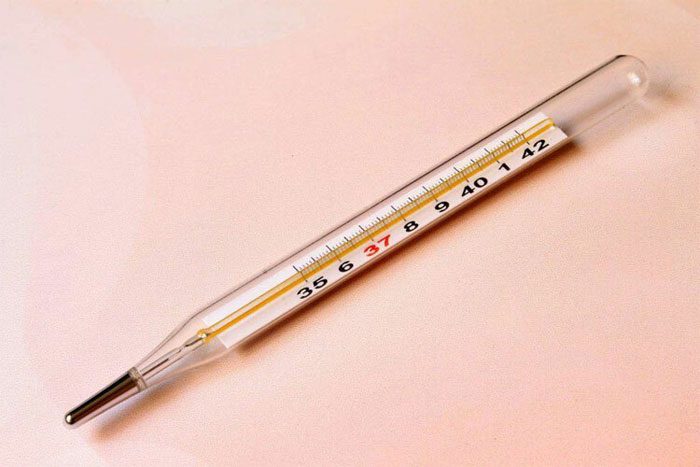
Mercury thermometer.
To enhance the usability of thermometers, the thermometric fluid must have two significant properties:
- First, the change in volume of the thermometric fluid in response to temperature changes must be very sensitive, allowing it to detect even very small temperature variations.
- Second, when measuring temperatures at low levels, the thermometric fluid should not solidify into a solid; conversely, at high temperatures, the fluid should not vaporize into gas. Otherwise, it cannot be used for temperature measurement.
For mercury and alcohol of equal mass, if we increase their temperatures by 1°C, experiments have found that the heat absorption of alcohol is significantly greater than that of mercury, approximately 20 times more. Therefore, the sensitivity of the volume change of the mercury column in a mercury thermometer is much greater than that of the alcohol column in an alcohol thermometer. In scientific experiments or when measuring human body temperature, the heat absorbed or released is very small, but it must reflect changes in temperature, so mercury thermometers are generally used. However, for the same temperature change, alcohol absorbs more heat and has a greater expansion capacity, making the fluctuations in the alcohol column more pronounced compared to the mercury column. When measuring the temperature of the surrounding air or water, alcohol thermometers are commonly used.
Alcohol and mercury have different characteristics: alcohol performs well in cold conditions, solidifying at –117°C, while mercury solidifies at –31°C, losing its fluidity. In cold regions, where winter air temperatures drop to about –40°C, using alcohol thermometers to measure air temperature is suitable. However, mercury has an advantage: it can withstand high temperatures better than alcohol. The boiling point of mercury is 356.72°C, while alcohol boils and vaporizes quickly at 78.3°C. In cases of measuring high temperatures, it is clear that mercury thermometers provide more accurate temperature measurements than alcohol thermometers.