We commonly recognize three states of matter: solid, liquid, and gas. The atoms in a solid are denser than those in a liquid, and the atoms in a liquid are denser than those in a gas. This is also why the solid form of a substance will sink when placed on the liquid form of that same substance.
Every time we drink ice water, we surely notice a familiar phenomenon: ice cubes usually float on the surface of the water. This can be puzzling—how can something as heavy as ice not sink?
In physics, we know that substances expand when heated and contract when cooled. If we apply this principle to explain the situation, at the same mass, ice at 0oC would have a smaller volume than water at room temperature. According to the formula for density, D = m/V, we can deduce that ice should have a greater density than liquid water.
So, logically, shouldn’t ice sink in water instead of floating? What is the reason behind this? Does it relate to buoyancy?

Why Does Ice Float on Water?
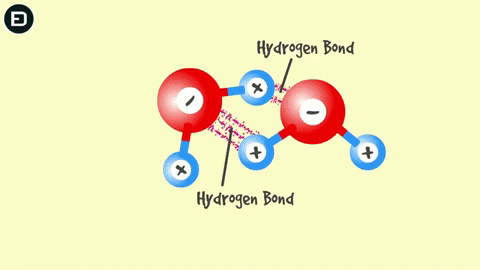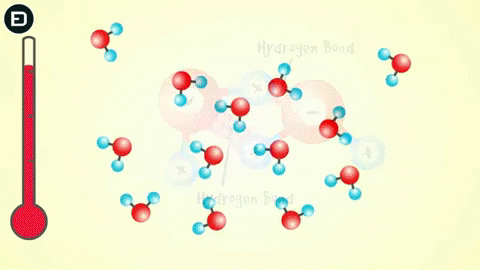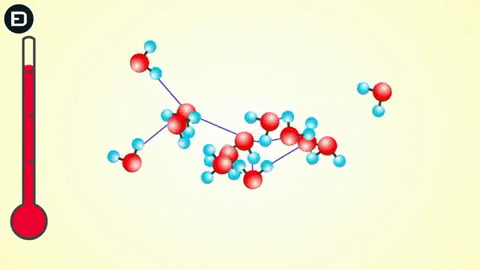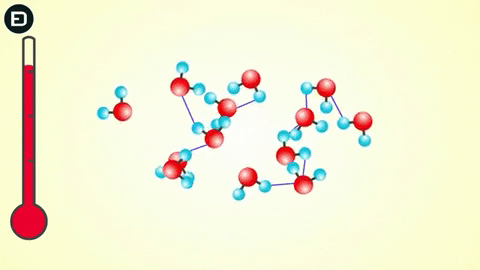
You might think that when liquid water begins to freeze, the molecules will bond more tightly together, but that’s not what really happens. Water has a unique interaction among its molecules that most other substances do not possess, called hydrogen bonding.

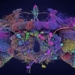
Water is composed of one oxygen atom bonded to two hydrogen atoms by covalent bonds, and this bond is pulled toward the oxygen atom, causing water to be polar. Consequently, water molecules can form hydrogen bonds.
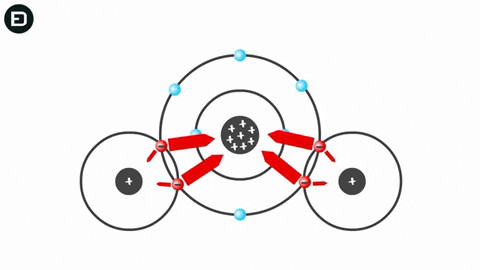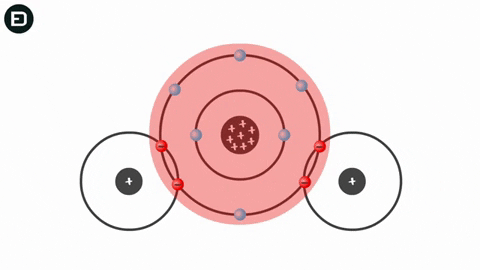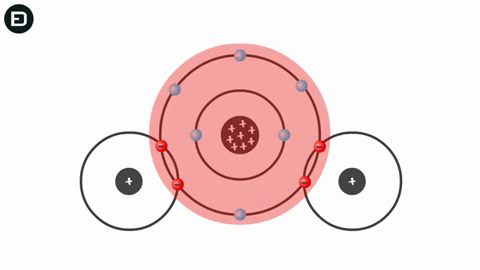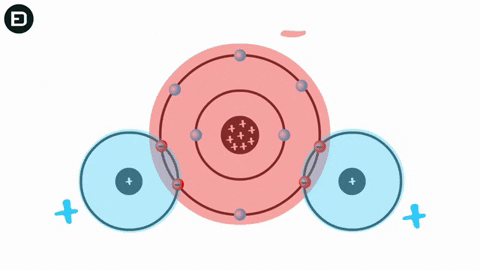
Experiments show that when the temperature is above 4oC, water molecules move rapidly, causing hydrogen bonds to break as the molecules collide due to thermal motion and electrostatic attraction. This means that the hydrogen bonds are not strong enough to hold the water molecules together.
However, when the temperature drops to 0oC, the water molecules slow down enough for the hydrogen bonds to connect, resulting in a change in the molecular structure of water to form a lattice.
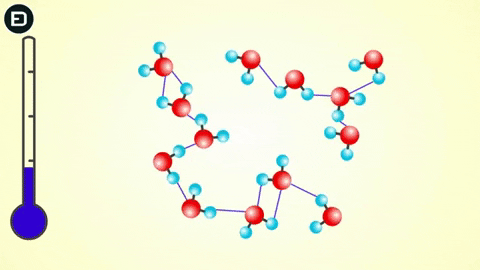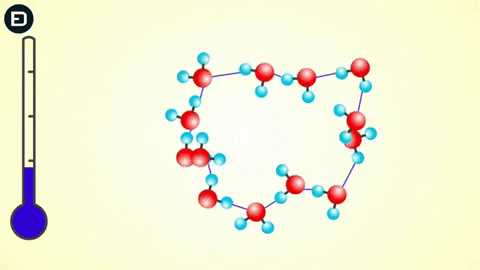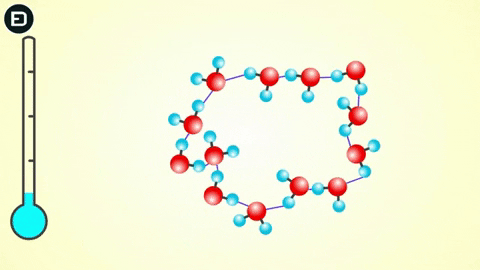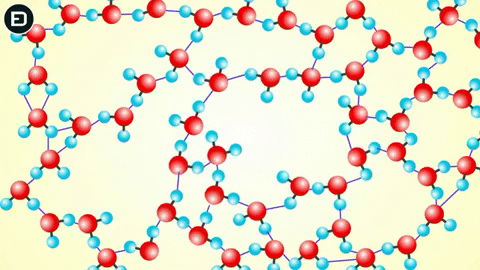
This orderly lattice structure is less dense than the disordered structure of liquid water. For this reason, the volume of ice increases when transitioning from liquid to solid, leading to the density of ice being less than that of liquid water. As you know, if an object is less dense than the liquid it is in, it will float.

What If Water’s Properties Were Like Most Other Substances?
A world without floating ice would mean that the ocean floors would freeze permanently. Bottom-dwelling creatures like lobsters, crabs, seaweed, and more would vanish. Forget about James Cameron’s Oscar because Titanic would never have sunk. And ultimately, we would say goodbye to our Arctic ice caps!