Chemists have developed a nanoscale structure capable of enhancing chemical reactions in the process of capturing carbon from the atmosphere.
Nanoscale structure combining copper, gold, and silver helps improve carbon capture. (Photo: Xinhua).
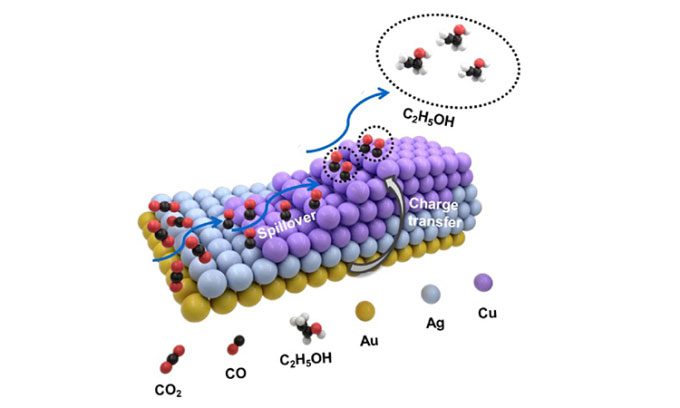
The research, led by Tianjin University and the Institute of Physics under the Chinese Academy of Sciences, utilizes a nanoscale structure combining copper, gold, and silver to function as a superior catalyst.
This asymmetric three-metal structure accelerates the electrochemical reduction of carbon dioxide, a crucial step for extracting carbon from the atmosphere and using it as a feedstock for industrial chemicals, according to a study recently published in the journal Nano Research.
The reaction employs electrical energy to convert greenhouse gases into other useful substances by separating carbon atoms from their oxygen counterparts.
The researchers altered the shape and ratio of the three metals by using “nanoscale pyramids” as “seeds” for further development, creating a unique form of heterogeneous structure.
Due to such structural differences, they were able to fine-tune the selectivity towards various C2-based products. For instance, the production of ethanol (C2H5OH) was maximized with a nanoscale structure consisting of one atom of gold and silver combined with five atoms of copper.