This is the 94th element in the periodic table (Pu) and is one of the most dangerous elements on Earth.
Plutonium is extremely rare, so rare that for many years we could not find it in the natural environment; the main source of plutonium is as a byproduct from nuclear reactors.
Plutonium is a radioactive substance, exhibiting a silvery appearance that tarnishes upon exposure to air. Queen Elizabeth II once held a piece of plutonium during her visit to the UK’s atomic energy research facility at Harwell in 1957; when she touched it, it felt quite warm and caused no visible pain or harm. Interestingly, even if you tried to taste or eat plutonium, it wouldn’t cause immediate harm; however, this is not recommended.

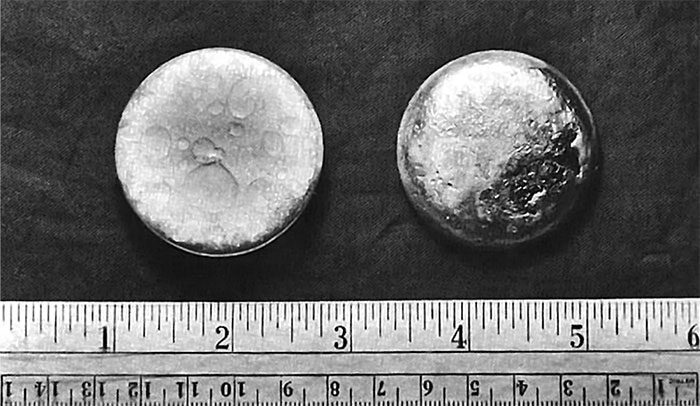
Plutonium is a radioactive substance.
Plutonium is a byproduct when uranium atoms are split in nuclear reactors; it is too large to penetrate our skin directly. However, the danger begins as human hands intervene.
One isotope of plutonium, known as plutonium-239 fissile, is listed by the United Nations as one of the only two radioactive isotopes used to manufacture atomic bombs. When neutrons collide with plutonium-239 atoms, fission occurs, releasing gamma rays and more neutrons. When these newly “born” neutrons collide with other plutonium-239 atoms, a similar reaction continues to occur repeatedly… releasing a massive amount of energy. Under suitable conditions, the energy from nuclear fission can be harnessed for various beneficial purposes for humanity. The heat generated can boil water to produce steam, which can turn turbines; one-third of the energy produced by nuclear power plants comes from plutonium.
In the past, this element was used as a weapon of mass destruction for warfare purposes. In 1945, the United States created a bomb containing just a small sphere of plutonium and dropped it on the city of Nagasaki, Japan, causing a tragic disaster that still haunts us today.

Plutonium-239 fissile is one of the two radioactive isotopes used to manufacture atomic bombs.
According to the International Panel on Fissile Materials (IPFM), established in 2006 and consisting of 17 countries, it is estimated that there are currently up to 140 tons of weapon-grade plutonium worldwide. This forms part of the so-called “nuclear threat” in countries such as the United States, the United Kingdom, and Russia. You only need an amount of plutonium the size of a bowling ball to create the core for an atomic bomb, yet gathering enough raw material is not easy. Plutonium does not naturally exist in large quantities; it primarily originates from nuclear reactors, and most plutonium is human-made. However, there is one place in Africa, Oklo in Gabon, where conditions were ideal for this element to form naturally.
Plutonium is one of the 15 actinide elements

Radioisotope Thermoelectric Generators (RTGs) are a type of nuclear battery that converts heat from decaying plutonium into electricity using wires called thermocouples. When one end of the thermocouple heats up, an electric current begins to flow. RTGs are used to supply power to remote locations, from lighthouses on treacherous coastlines to space probes exploring the vast universe. However, you cannot use just any old radioactive element to create them; NASA has a strict list of criteria for nuclear batteries. The fuel must be safe in case of an accident, releasing minimal beta, gamma, or neutron radiation, as these can interfere with equipment. It must be as stable as possible because you wouldn’t want it to explode unexpectedly, and it also requires a sufficiently long half-life since batteries cannot be replaced in space.

In 1940, scientists at the Berkeley Radiation Laboratory experimented with uranium, bombarding it with heavy hydrogen atoms to see what would happen. Uranium is a typical radioactive substance, and this bombardment caused it to split, subsequently releasing another radioactive element – neptunium. However, this element is unstable and decays into another radioactive element that had never been seen before. Following the theme of “planets,” scientists named it plutonium. With its promising nuclear weapon potential, within just two years, the highly classified process of plutonium production began at the Metallurgical Laboratory in Chicago.

Some Applications of Plutonium
























































