What Happens If a Gold Sheet is Only One Atom Thick?
For centuries, jewelers of all eras have sought ways to create gold in increasingly intricate shapes. However, achieving an ultra-thin gold layer has always been a challenge due to technological limitations.

The successful creation of a new form of gold that is only one atom thick. (Illustration: Getty).
Until now, a method based on modern chemistry has finally produced a form of gold material that cannot be made thinner, with a thickness of just one atom.
This is considered a breakthrough in the materials industry. “If you create an extremely thin material, something extraordinary will happen, just like with graphene,” says Shun Kashiwaya, a materials scientist at Linköping University in Sweden.
It also possesses some intriguing properties never before seen in three-dimensional forms of gold. According to Kashiwaya, similar phenomena observed with graphene—the lightest material in the world—might also occur with gold.
“As is known, gold is considered a metal, but if it is only one atomic layer thick, gold can become a semiconductor”, Kashiwaya explains.
Adhering to the naming conventions of material science, the researchers decided to name this new two-dimensional material goldene.
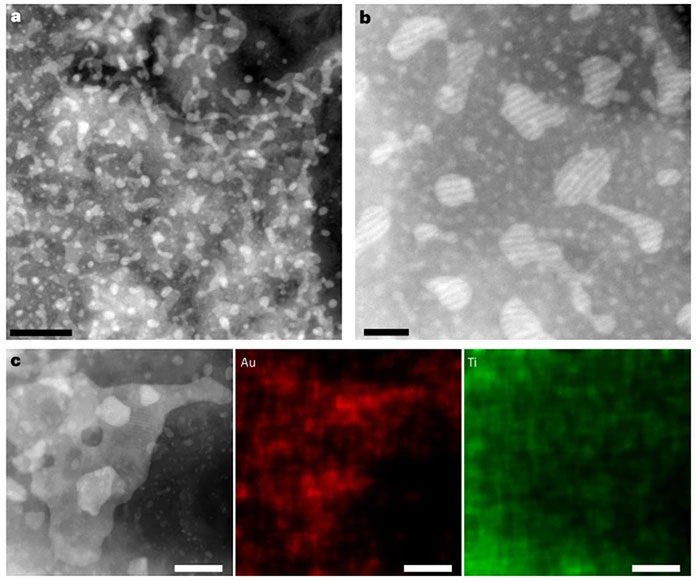
The new form of gold observed under a microscope. (Photo: Nature Synt).
What makes goldene special is that gold has long been considered very difficult, if not impossible, to form a two-dimensional configuration, due to its tendency to clump together.
Previous efforts succeeded in creating thin gold sheets just a few atoms thick or a single layer sandwiched between other materials.
Experts note that gold is inherently a very good conductor of electricity. However, if the particles are in a two-dimensional form like in this new material, the atoms will have two free bonds, enabling them to gain additional properties to function as semiconductors—capable of conducting electricity between conductors and insulators.
This capability is highly beneficial for related manufacturing industries, as the conductivity of the material can be adjusted to suit specific applications.
Combining this material with the properties of semiconductors also opens up entirely new ways that we can apply it, such as in water filtration, communication, and chemical production.


















































