For decades, many researchers have pursued the idea that: eating restrictions, calorie reduction, and even planned fasting are the keys to a healthy life and long longevity.
Now, they have found evidence supporting this effect. In a new study examining the impact of fasting on mice, scientists discovered that a prolonged fasting period of 24 hours likely activated a metabolic switch, promoting the regeneration of stem cells in the intestines.
These intestinal stem cells diminish as we age and fail to regenerate effectively thereafter.
Notably, intestinal stem cells play a crucial role in helping us maintain healthy tissues and fight diseases. Therefore, if fasting for one day can reactivate the regeneration of these stem cells, it would be a miraculous effect worth investigating.
The study was published in the journal Cell Stem Cell.
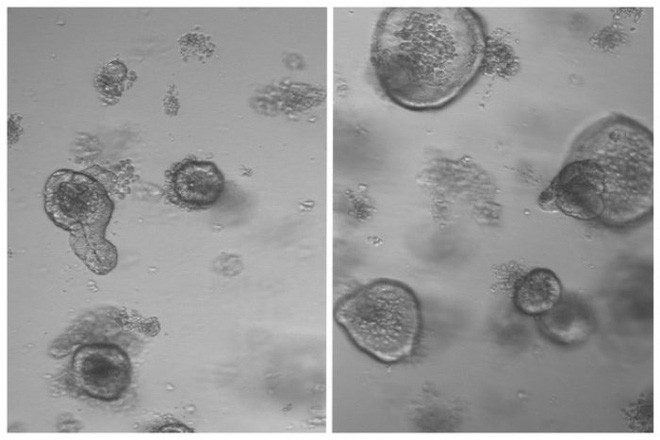
24-hour fasting enhances stem cell regeneration in the intestines of mice.
“Fasting significantly affects the intestines, including enhancing the regeneration processes within this organ, as well as increasing the ability to combat any disease that invades the intestines, such as infections or cancer,” said biologist Omer Yilmaz at the Massachusetts Institute of Technology (MIT).
“This study provides evidence that fasting induces a metabolic change in intestinal stem cells, shifting from carbohydrate usage to fat burning.”
This change not only means that the cells will use fat as an energy source instead of carbohydrates, but it also enhances cellular function.
Scientists refer to intestinal stem cells as “workhorses,” implying that they have significant roles and serve the function of the intestines and the body throughout life.
Typically, these cells regenerate the intestinal lining every five days. However, when fasting triggers a metabolic shift, the regeneration process can occur more rapidly.
In the laboratory, Yilmaz’s research team extracted intestinal stem cells from mice that had fasted for 24 hours and cultured them in a nutrient environment to form intestinal-like cell clusters called organoids.
In doing so, they found that the regenerative capacity of stem cells taken from mice that fasted for one day was twice that of normal mice that did not fast.
According to medical researcher Maria Mihaylova, a member of the research team: “It is clear that fasting has a substantial impact on the ability to form organoids from stem cells. This is something we have observed in both young and old mice, and we really want to understand the molecular mechanisms driving this.”
To investigate further, Mihaylova’s team decoded the messenger RNA of stem cells from fasting mice and found that 24 hours of fasting activated transcription factors known as peroxisome proliferator-activated receptors (PPAR). This receptor activated genes involved in fatty acid metabolism.
In this case, the activation led the cells to break down fatty acids instead of glucose, thereby enhancing regenerative ability. When they blocked PPAR activation, the enhanced regeneration eventually ceased.
But that is not all that the research team discovered. By injecting a molecule called GW501516 into the mice to activate PPAR, this drug could replicate some of the beneficial effects of fasting in mice.
“That was also quite surprising,” said Chia-Wei Cheng, one of the researchers. “Just activating this one metabolic pathway is sufficient to reverse certain aging phenotypes.”
If there were a drug that could mimic the effects of fasting, it could assist those, often patients, who cannot undertake this practice. For example, elderly individuals and cancer patients after chemotherapy who are physically weak are unable to fast to trigger health effects, even though they desperately need them.

Numerous studies have shown the health benefits of fasting.
Of course, these are just preliminary results. There is much more for researchers to explore before we fully understand the extent of this metabolic switch and its operational functions—Can this effect be easily activated in humans as it is in mice? Do you only need to fast for 24 hours to regenerate your intestines?
These questions remain unanswered. However, as the issue becomes clearer, we will stand before an opportunity to develop medications that promote intestinal function and overall health, helping to extend longevity.
“This work aligns with a rapidly growing field, demonstrating that nutrition and metabolism profoundly influence cellular behavior, which may relate to human diseases,” said biochemist Jared Rutter from the University of Utah, who was not involved in the study.


















































