Scientists have observed a self-healing metal for the first time, which could be applied in various engineering fields from road repairs to mobile phones.
A research team from Sandia National Laboratories and Texas A&M University tested the durability of the metal using a specialized transmission electron microscopy technique to stretch the metal at both ends 200 times per second. They then observed the self-healing capability at the nanoscale on a 40-nanometer thick platinum piece suspended in a vacuum chamber.
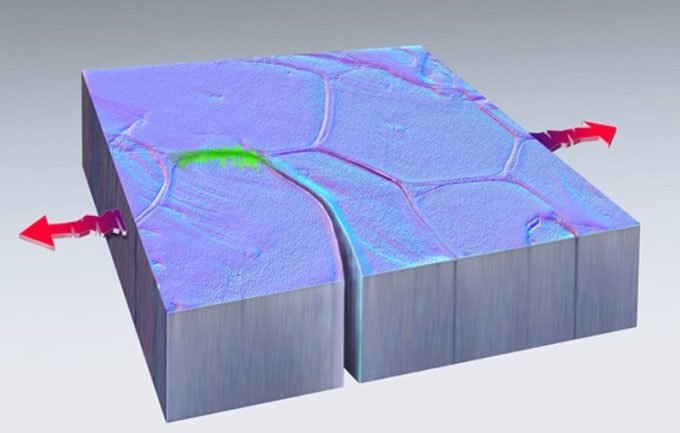
Platinum block being stretched at both ends in the experiment. (Photo: Dan Thompson)
The cracks caused by the tension described above are referred to as fatigue failure. This is pressure and repetitive motion that leads to microcracks, ultimately causing machinery or structures to fracture. After about 40 minutes of observation, researchers noted that the cracks in the platinum began to close and self-repair before starting to fail in another direction. They published their findings on July 19 in the journal Nature.
“We did not expect this. What we can confirm is that metals have the ability to self-heal, at least in the case of fatigue failure at the nanoscale,” said materials scientist Brad Boyce at Sandia National Laboratories.
Although the research team is still unclear about the exact process and how to utilize it, the self-healing metal will undoubtedly make a significant difference in repairing everything from roads to mobile phones.
In 2013, materials scientist Michael Demkowicz at Texas A&M University worked on research predicting that the process of healing nano-cracks could occur due to tiny crystal grains within the metal moving boundaries to respond to pressure. Demkowicz also participated in the new research, using updated computer models to validate his previous hypothesis about the self-healing behavior of metals at the nanoscale, aligning with what was observed in the experiment.
This automatic repair process occurring at room temperature is another promising aspect of the research. Metals typically require a lot of heat to change shape, but the experiment took place in a vacuum environment. Researchers need to consider whether a similar process occurs in regular metals in everyday environments.
A feasible hypothesis includes the process of cold welding, which occurs when metal surfaces are close enough for their atoms to bond with each other. Usually, a thin layer of air or contaminants interferes with this process. In environments like outer space, pure metals can be pressed close enough together to bond securely.




















































