Throughout the long history of humanity, gold has been pursued by many civilizations for its value. With the advancement of the era and technological progress, to this day, there still isn’t a feasible and economical process for synthesizing gold.
The Challenges of Synthesizing Artificial Gold
The synthesis of artificial gold is a very challenging task because the atomic structure of gold is very stable and has a high-energy electronic configuration. This makes the electron arrangement of gold very compact, complicating efforts to alter the atomic structure of gold through chemical reactions.
To synthesize artificial gold, scientists utilize high temperature and pressure conditions. High temperatures provide sufficient thermal energy to break the bonds between gold atoms, causing the atoms to rearrange. High pressure can provide enough force for the atoms to combine into the crystalline structure of gold under high-temperature conditions.
Currently, scientists primarily employ two methods to achieve the synthesis of artificial gold: nuclear transmutation and atomic approval. Nuclear transmutation is the process of changing the atomic nucleus of an element into gold, often requiring higher temperature and pressure conditions. Atomic approval involves achieving gold synthesis by injecting other elements among the atoms to alter the electronic structure of gold.
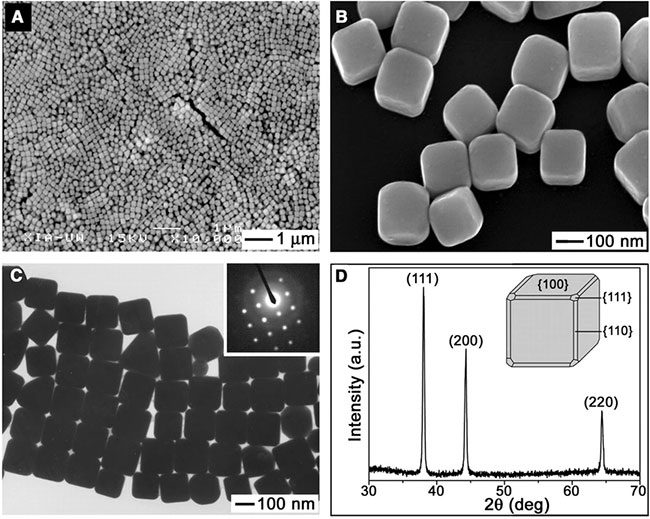
Synthesizing gold requires high-energy reaction conditions, making traditional synthesis methods overly costly. Traditional synthesis methods primarily involve high-temperature, high-pressure techniques and solvent methods, consuming a lot of energy and also producing environmental pollutants.
However, it is worth noting that synthetic gold is not a solution for wealth and prosperity. On one hand, the process of synthesizing artificial gold is very complex and requires multiple-stage reactions under high temperature and pressure conditions, making the production process time-consuming and energy-intensive. On the other hand, there is a quality difference between synthetic gold and natural gold; its purity is not as high as that of natural gold, so it cannot be sold on the market at a price comparable to that of natural gold.
Although the technology for synthetic gold still faces many challenges, scientists remain optimistic as they explore this field. As technology continues to advance, we may see more advanced methods proposed, and perhaps one day, the synthesis of gold will become a viable option.
In recent years, several new synthesis methods have been proposed in an effort to reduce the energy required to synthesize gold. For instance, low-temperature-low-pressure methods are used to synthesize gold at relatively low temperatures and pressures by controlling the synthesis conditions. This approach may reduce energy consumption and mitigate environmental pollution issues.
The Inability to Fully Grasp the Preparation of Metal Phases
One of the difficulties in metal phase preparation lies in the selection and preparation of raw materials. Gold exists in nature as gold ore, mixed with many other metals and impurities. To carry out the artificial gold synthesis process, impurities and other metals in the raw materials must first be removed through steps such as enriching and refining the ore to obtain high-purity gold.
The preparation of metal phases is also limited by preparation conditions. Temperature, pressure, reaction time, and environmental air all play crucial roles in the metal phase preparation process. The high melting point and low reactivity of gold make the conditions for its synthesis more challenging and complex. However, the limitations concerning the understanding of the metal phase preparation process remain inadequate, and it is impossible to determine the most suitable preparation conditions, which increases the difficulty of the artificial gold synthesis process.

The process of synthesizing gold requires catalysts to facilitate reactions. However, an effective and economical catalyst for the artificial gold synthesis process has yet to be discovered.
The preparation of metal phases requires precise control over temperature, pressure, and other preparation conditions, while the demands for laboratory and industrial production equipment and technology are very high. Currently, although the development of science and technology has brought numerous new techniques and experimental tools, the preparation of fully phased metals still faces many challenges.
While humanity has yet to fully grasp all phases of gold preparation, scientists have been striving to overcome this challenge and realize the process of synthesizing artificial gold. This not only contributes to the development of the metallurgy industry but may also bring revolutionary changes in the field of gold applications.
Challenges in Gold Synthesis
Synthetic gold has always been one of the dreams of the chemistry community but faces many challenges. First, the process of synthesizing gold involves complex chemical reaction pathways. The chemical synthesis process of gold requires the reduction of other metal ions to metallic gold and the formation of gold crystals. This process involves multiple reaction steps and the formation of intermediate products.
The synthesis of artificial gold includes many reaction details, such as reaction time, temperature, air, etc. Hence, optimizing these reaction conditions is another challenging issue to achieve cost-effective gold synthesis.
One of the key reaction steps is the reaction of metal ions with a reducing agent to form metal particles. However, this reaction itself is very complex, and scientists face the challenge of various non-linear and competitive reactions. Therefore, finding an effective and controllable reaction pathway is one of the primary problems in realizing the process of synthesizing artificial gold.
The development of gold crystals is also a very challenging task. Gold crystals are composed of many small gold particles that gradually synthesize and form crystals through a certain growth mechanism. However, in practical synthesis, it is often difficult to control the size, shape, and orientation of gold crystals. This is due to the development of crystals being influenced by many factors, including reaction conditions, solution concentration, temperature, stirring speed, etc. Scientists need to carefully adjust these conditions to obtain ideal gold crystals. However, the complexity and unpredictability of this process make it challenging to accurately control the gold crystals.
To address these challenges, scientists have conducted extensive research. They strive to achieve efficient gold synthesis by adjusting intermediates and catalysts in the reaction process. Simultaneously, they propose a series of reaction conditions and new synthesis strategies to improve control over crystal development. For example, scientists have discovered that varying the reaction temperature and stirring speed can significantly impact the morphology of gold crystals. Additionally, they utilize surfactants to control the size and shape of gold particles.


















































