The search for oxygen in the atmosphere of a planet is a clue that life may be occurring.
On Earth, photosynthetic organisms absorb carbon dioxide, sunlight, and water, producing sugars and starches for energy. Oxygen is a byproduct of this process, so if we can detect oxygen on another planet, it would undoubtedly excite the scientific community.
However, researchers have raised questions about the idea that the presence of oxygen in the atmosphere of an alien planet would be a sign of life. It is only evidence of life if we can rule out other pathways that could produce oxygen.
Earth is saturated with oxygen. It makes up 46% of the crust and the same percentage of the mantle, while the atmosphere contains about 20% oxygen.
The presence of oxygen originated from the Great Oxygen Event (GOE) around two billion years ago. Ancient cyanobacteria evolved pigments that absorbed sunlight and used it in the process of photosynthesis. Oxygen is a waste product of photosynthesis, and life existed for billions of years before oxygen was created in the atmosphere, mantle, and crust.
Therefore, if scientists find oxygen in the atmosphere of an extraterrestrial planet, it suggests that life may be active. Simple life forms could be thriving in the oceans of the planet, absorbing sunlight and releasing oxygen.
But new research has identified a source of oxygen that does not depend on life.
The research paper titled “Abiotic Molecular Oxygen Production — The Ion Pathway from Sulfur Dioxide” was published in the journal Science Advances. The lead author is Måns Wallner, a physics doctoral student at the University of Gothenburg in Sweden.
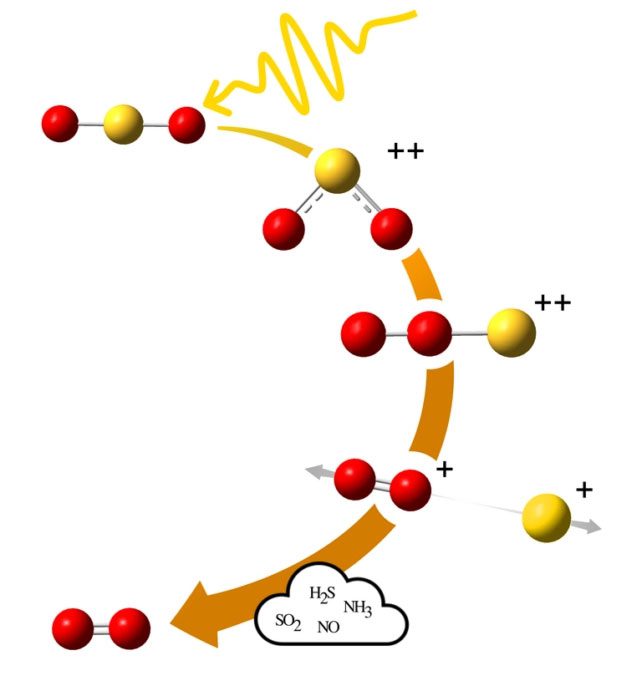
Researchers have discovered an abiotic source of oxygen derived from sulfur dioxide. Sulfur is not rare in celestial bodies, and since volcanoes produce sulfur and release it into the atmosphere, extraterrestrial planets could have oxygen in their atmospheres without the need for life.
Instead, high-energy radiation from a star can ionize sulfur dioxide molecules. The formula for sulfur dioxide is SO2, and when it is ionized, the molecule rearranges itself. It becomes a “doubly positively charged system”. It then takes on a linear form with both oxygen atoms at one end and sulfur at the other. This is referred to as rearrangement, as the oxygen atoms freely tumble in chaotic orbits until they settle into new compounds.
“When doubly ionized, two of the bonding electrons in the molecule are pushed out, which can lead to a change in the angle between the atoms in the molecule,” lead author Wallner stated in a press release.
“Additionally, it is also important in the current case that rearrangement can occur, meaning that the atoms swap positions and the molecule takes on a completely new shape.”
However, the components of the molecule may not revert back to SO2. Instead, sulfur may break apart, and a simple positively charged oxygen molecule could still exist. The positive charge could then be neutralized by attracting an electron from another molecule.
This pathway to oxygen may explain some of the oxygen we find in other locations. Io, Ganymede, and Europa all have oxygen in their atmospheres, and rearrangement could be the cause.
Io is home to many volcanoes — the most volcanically active body in the Solar System — so life is excluded there. Ganymede and Europa have subsurface oceans, making them potential hosts for life. However, that life cannot build an oxygen atmosphere like life on Earth. An alternative explanation is needed to account for the oxygen found on these moons.
According to the researchers, this oxygen pathway could also occur on Earth. Raimund Feifel, co-author of the paper, stated: “We also propose in our paper that this occurs naturally on Earth.”
This ion formation pathway could also work for other molecules, and that is the next step for researchers. They want to see if other molecules, such as carbon diselenide, can undergo double ionization.
Feifel noted: “We want to see if that occurs subsequently, or if it is just a coincidence with sulfur dioxide.”
A 2014 paper presented evidence of molecular oxygen generated from CO2 when exposed to high-energy UV radiation.
In a 2015 paper, Japanese researchers showed that near-ultraviolet light could produce O2 on planets when interacting with water using Titania (titanium dioxide) as a catalyst.
These findings help explain how Earth had a small amount of oxygen in its atmosphere before the GOE. The James Webb Space Telescope is part of the context for this research. Studying exoplanet atmospheres is one of the scientific goals of the telescope, and with its powerful infrared tools, it is poised to reveal the chemical makeup of exoplanet atmospheres.





















































