The Destructive Power of Atomic Bombs (Nuclear Bombs) Originates from Tiny Particles: Protons, Neutrons, and Electrons.
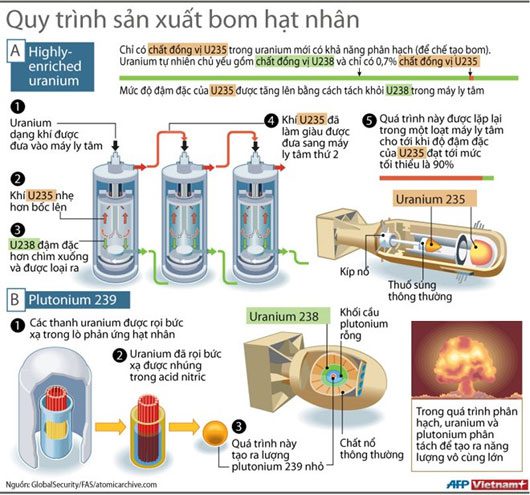
Isotope U-235 was selected due to its very high capability to absorb free neutrons. The process of absorption and decay occurs extremely quickly.
For it to function, uranium fuel must be “enriched,” meaning the proportion of the U-235 isotope must be increased. At the nuclear weapon level, the U-235 isotope must account for over 90% of the primary material.
Only the U-235 isotope in uranium has the capability for fission (to create bombs). Natural uranium mostly consists of the U-238 isotope, with only 0.7% being U-235.
The structure of a simple fission bomb includes a small U-235 warhead and a spherical mass of U-235 material; when the warhead meets the mass, it creates a critical mass and triggers the chain reaction process of fission.