Scientists have discovered a new series of materials that are “superior” to graphene. This is predicted to revolutionize the battery manufacturing sector.
According to Slash Gear, researchers have predicted a “new carbon network,” similar to graphene but with a much more complex microstructure. This discovery could lead to the development of better electric vehicle batteries. Graphene – a form of carbon regarded as one of the most extraordinary materials – is considered a game-changer in lithium-ion battery technology. However, the newly discovered material has the potential to create battery cells with higher energy density.
Graphene is essentially a network of carbon atoms, consisting of small hexagons formed when each hexagon bonds with three neighboring hexagons (creating a honeycomb-like structure). However, scientists have hypothesized that carbon atoms can also bond in ways that produce structures different from graphene’s simple honeycomb form.
This is what the research team from Marburg University in Germany and Aalto University in Finland has developed. Once again, scientists are harnessing carbon atoms but in a new direction. They created what is called “Biphenylene Network” – a result formed from square and octagonal bonds in a more complex network than graphene’s structure. This new structure possesses different electronic properties and has several advantages over graphene.
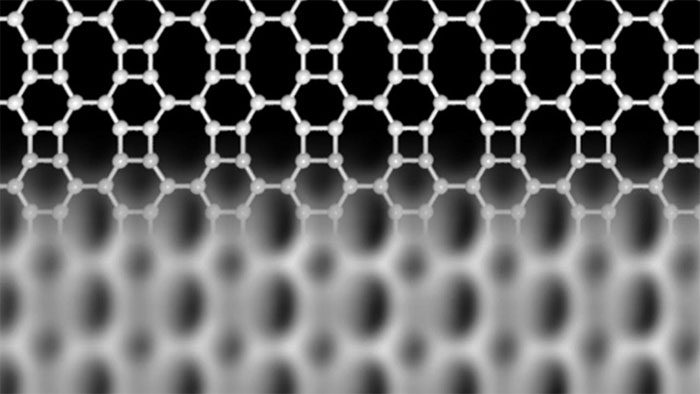
The new carbon network is similar to graphene but has a much more complex microstructure.
For example, while graphene is highly valued for its ability to function as a semiconductor, the new carbon network behaves more like a metal. In fact, with a width equivalent to just 21 atoms, the stripes of the Biphenylene network can act as conductors for electronic devices. In contrast, graphene still functions as a semiconductor at a similar scale.
Qitang Fan from Marburg University, the lead author of the new study, stated: “This new carbon network could also serve as a high-performance anode material in lithium-ion batteries, with greater lithium storage capacity than currently used graphene-based materials.”
The anode of lithium-ion batteries typically consists of graphite layered on a copper foil. It has high electrical conductivity, which results in an efficient and long-lasting battery.
However, a smaller, more efficient alternative based on this new carbon network could create cells with denser configurations. This could allow electric vehicles and other devices to use smaller and lighter lithium-ion batteries.
Compared to graphene, the next challenge is finding a way to produce this new version on a large scale. The current bonding method relies on an ultra-smooth gold surface, on which carbon-containing molecules initially form hexagonal chains that bond together. Following this, a reaction will bond these chains into squares and octagons to create a structure different from graphene.
Linghao Yan from Aalto University explained: “The new idea is to use refined molecular precursors to create biphenylene instead of graphene.” The goal now is to produce larger sheets of the material to better understand its properties before considering practical applications.



















































